Abstract
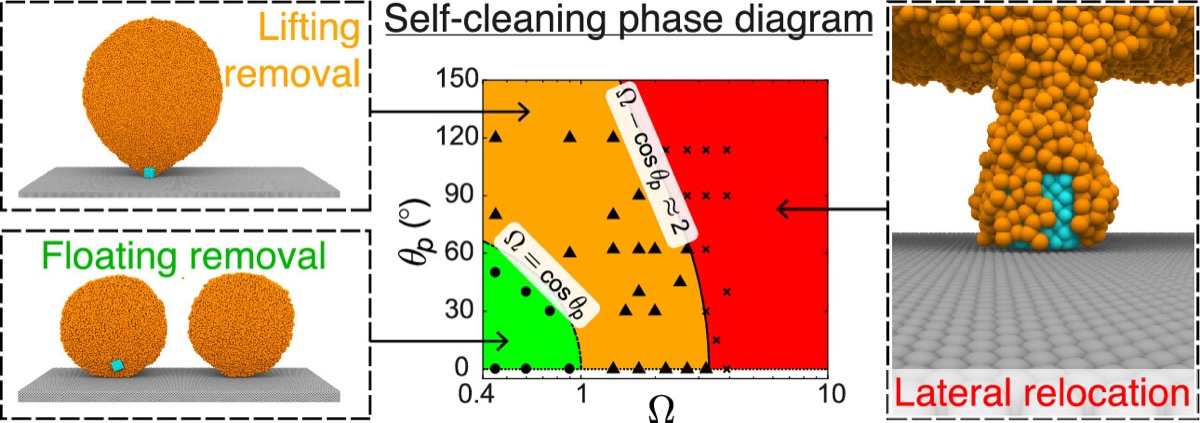
Many organisms in nature have evolved superhydrophobic surfaces that leverage water droplets to clean themselves. While this ubiquitous self-cleaning process has substantial industrial promise, experiments have so far been unable to comprehend the underlying physics. With the aid of molecular simulations, we rationalize and theoretically explain self-cleaning mechanisms by resolving the complex interplay between particle–droplet and particle–surface interactions, which originate at the nanoscale.